Home » How to Recycle and Reuse Aluminum Foil: Can You Recycle Aluminum Foil?
Does Silicone Have Microplastics?
Written by Admin | Nov 20, 2025

What Is Silicone and How It Differs from Plastic
Silicone Chemistry 101 (Polysiloxanes, Cross-linking)
Silicone is a family of polymers built on a silicon–oxygen (Si–O–Si) backbone with organic side groups. Depending on how it’s cross-linked (e.g., platinum-cured addition systems or peroxide-cured systems), you get everything from soft elastomers (bakeware, pacifiers) to sealants and oils. Cross-link type affects odor, residual volatiles, and high-temperature stability—which is why premium food-contact goods often highlight platinum curing.
Structural & Performance Differences vs. Conventional Plastics
Compared with common plastics (PE, PP, PVC), silicone’s Si–O backbone gives excellent thermal stability and chemical inertia—one reason it’s used from freezer to oven and in medical applications. Still, performance depends on recipe and curing; poorly post-cured items may outgas more at first use.
Quick comparison (for context)
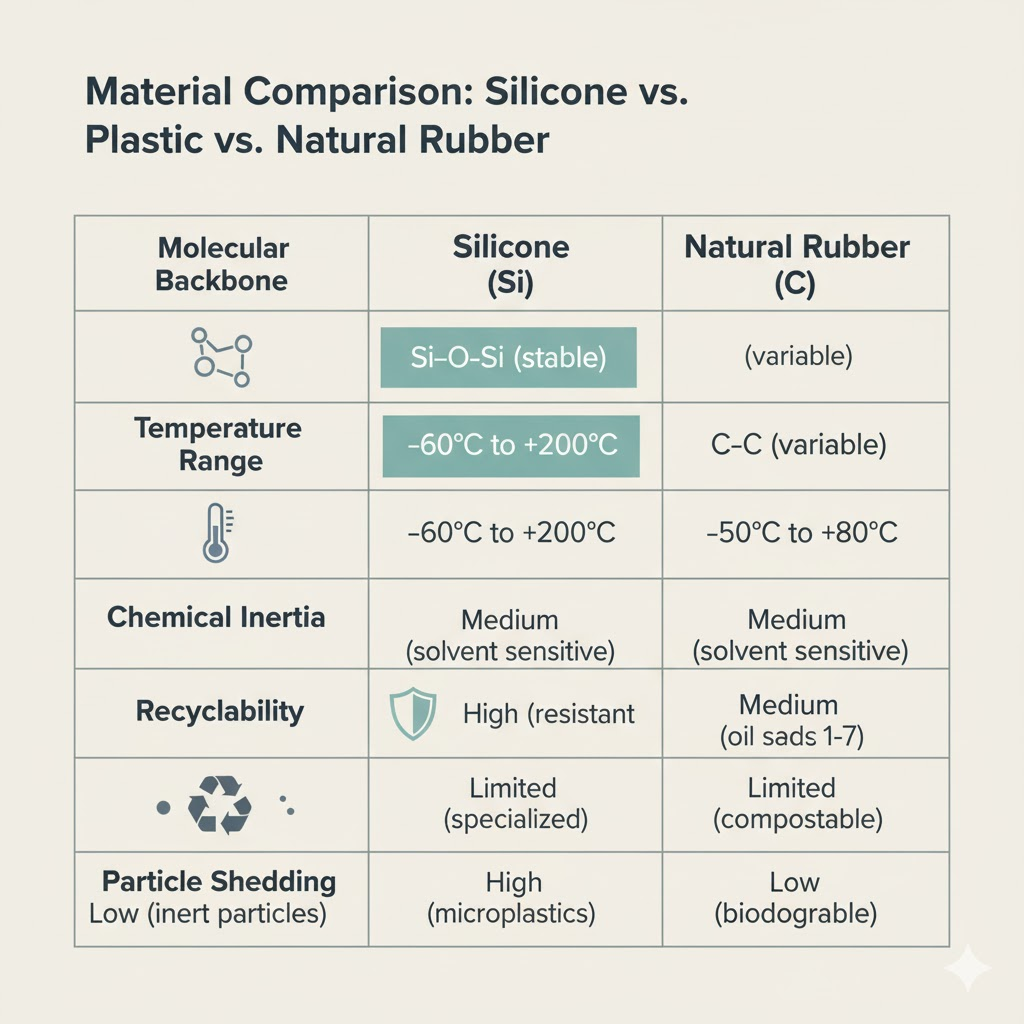
| Property / Material | Silicone (polysiloxane) | PE/PP (polyolefins) | Natural Rubber (NR/Latex) |
| Backbone | Si–O–Si | C–C | C–C with unsaturation |
| Typical temp range | ~-60°C to 200–230°C (per product label) | ~-20°C to 100–120°C | ~-50°C to ~90°C |
| Chemical inertia | High | Moderate (depends on solvent/temp) | Moderate |
| Common food-contact rules | 21 CFR 177.2600; LFGB/BfR | 21 CFR parts for plastics; EU (Reg. 10/2011) | Varies; not typical for high-heat cookware |
| Micro/nano particle tendency* | Low under normal use; ↑ with abrasion/aging | Can fragment under UV/heat/mechanical stress | Can shed; some particles show aquatic toxicity in studies |
| End-of-life | Not biodegradable; limited recycling | Some recyclable (infrastructure varies) | Not recyclable; biodegradable under specific conditions |
*Tendency refers to relative risk based on lab observations; real-world outcomes depend on quality, use, and care.
(Optional visual ideas to add later for readers and SEO images):
- Diagram: Si–O–Si vs C– C backbones (simple labels, descriptive alt text).
- Mini flowchart: “Post-cure in 4 steps” (mirrors the How-To below).
- Icon row: “Shedding drivers—abrasion > UV/heat.”
Are Silicone Fragments Considered “Microplastics”?
Microplastics & Nanoplastics: Definitions and Size Ranges
Microplastics are usually defined by size (≤5 mm) and insolubility; many frameworks now reference nanoplastics for the <1 µm range. The EU’s 2023 restriction targets “synthetic polymer microparticles,” explicitly size-bounded, water-insoluble, and persistent.
Where Silicone Fits Within Regulatory Definitions
Because silicone is a synthetic polymer, any persistent, water-insoluble silicone fragments in the regulated size range can be treated as “microplastics” under EU rules—even though their chemistry differs from petro-plastics. That’s a regulatory classification, not a claim that silicone behaves like polyethylene in the environment.
Plain-English tie-in: if a tiny silicone fragment is synthetic, water-insoluble, and persistent, regulators may classify it in scope—even if it’s not a petro-plastic.
Does Silicone Release Micro- or Nanoparticles?
What Laboratory Studies Show (Pacifiers, Sealants, Appliances)
Pacifiers: In controlled tests that simulate mechanical wear, silicone pacifiers released nanoparticles (mean sizes around ~130–180 nm depending on method). Boiling alone didn’t shed detectable particles, but pre-boiling increased subsequent release under abrasion. Latex pacifiers also shed particles, which were acutely toxic to Daphnia magna—silicone particles were not in that assay.
Kitchen sealant: Raman imaging + SEM captured silicone micro- and nanoplastics from real and mimicked kitchen sealant, including from aged seams. The work pushed Raman mapping near/under the diffraction limit using image reconstruction.
Triggers for Particle Shedding: Heat, UV, Abrasion, Aging
The main drivers are mechanical abrasion and aging/weathering; UV and repeated thermal cycling can exacerbate wear in many polymers. In the pacifier study, boiling did not directly shed particles but primed the silicone so later abrasion released more. Sealant work likewise points to handling, scraping, and long-term use around sinks/roofs.
Particle Sizes, Counts, and Detection Methods (Raman/FTIR, NTA, DLS)
Researchers identified silicone particles using ATR-FTIR spectral fingerprints and imaged them with TEM/SEM; NTA and DLS quantified sizes and distributions, with Raman imaging mapping composition down to ~100 nm via advanced processing.
Methods at a glance (reader-friendly)
- Raman imaging: maps a polymer’s “spectral fingerprint” across an area to find tiny particles—and confirms they’re silicone rather than dust.
- ATR-FTIR: checks the chemical bonds (e.g., Si–O–Si, Si–CH₃) to verify silicone composition.
- NTA/DLS: estimate particle size/distribution in liquids; each has different sensitivities and assumptions.
- TEM/SEM: visualizes particle shapes/sizes at very high magnification.
For Advanced Readers: Method Nuances
- Raman limits: Resolution is diffraction-limited; studies use deconvolution/image reconstruction to flag sub-300 nm features responsibly.
- NTA vs DLS: NTA is number-weighted and sensitive to bright, moving particles; DLS is intensity-weighted and biased toward larger agglomerates.
- ATR-FTIR: Confirms silicone chemistry; spectra of fragments closely match bulk PDMS signatures.
- Controls matter: Background contamination and instrument wear (e.g., blender shedding) are controlled in robust protocols.
Particle Shedding vs. Chemical Migration
Additives, Curing Residues, and Volatile Siloxanes
Beyond solid fragments, volatile siloxanes and other compounds can migrate from some silicone bakeware—particularly when post-curing/tempering is insufficient. A multi-country consumer test found 23% of molds released high or increasing volatiles across repeated uses; repeated heating typically reduces emissions as residues are driven off.
How Migration Differs from Physical Fragment Release
Migration concerns molecules (e.g., cyclic VMS like D4/D5/D6) that may enter food/air during heating; shedding concerns solid particles detached by wear. They pose different risks, are measured with different methods, and are mitigated by different actions (e.g., post-curing vs. gentle handling).
Health & Safety in Everyday Use
Food-Contact Silicone: Certifications and Standards (FDA, LFGB)
In the U.S., many silicone elastomers used for repeat food contact fall under 21 CFR 177.2600, which also sets extractives limits; EU-wide, silicone isn’t yet harmonized like plastics, so authorities often rely on BfR Recommendation XV (Silicones) and national rules to judge safety. Reputable brands disclose FDA/LFGB compliance and often post-cure parts to minimize volatiles.
High-Heat Cooking, Steaming, and Sterilization Considerations
Normal oven use is what silicone is designed for; pre-heating new bakeware empty for a cycle or two (or washing and baking with no food) can help drive off residual volatiles, reducing odors and migration on first use. Consistently staying within the labeled temperature range and avoiding direct flame improves longevity and keeps emissions lower.
Infant Products (Pacifiers, Nipples): Special Precautions
For baby items, follow the maker’s boil/steam sterilization instructions and inspect frequently. The pacifier study suggests boiling doesn’t itself shed particles, but post-boil abrasion can increase release—so replace at the first signs of tearing, stickiness, or whitening.
When to Replace Silicone Items
Retire pieces that feel tacky after washing, discolor unusually, develop cracks/tears, or hold a persistent odor even after deep-cleaning. These are practical signals the polymer has aged or was under-cured initially.
Environmental Impact of Silicone
Durability and Reuse Benefits
Silicone’s durability can replace dozens of disposables (liners, bags, gaskets), reducing waste when products are used for years—its main environmental upside versus lower-lifespan plastics. (This is also why quality and care matter.)
Everyday example: One silicone baking mat can replace ~100 sheets of parchment in a typical home kitchen per year.
Biodegradability and Persistence
Silicone does not biodegrade in typical conditions; environmental breakdown is slow. Some studies describe chemical degradation pathways (e.g., hydrolysis to dimethylsilanediol under certain conditions), but in real-world settings silicone behaves as a persistent material.
End-of-Life Options: Recycling Pathways and Challenges
Curbside recycling rarely accepts silicone. Some brands run take-back programs (e.g., TerraCycle partnerships for specific silicone products). On the R&D front, chemical recycling is making progress, depolymerizing silicones back to cyclic monomers, though industrial scale is still emerging.
How to Minimize Particle Shedding

Buying Tips (Platinum-Cured, Filler-Free, Reputable Brands)
Choose 100% silicone from reputable makers, ideally platinum-cured for food contact; look for clear FDA/LFGB statements and instructions that include post-curing/tempering steps. Avoid chalky, strongly odorous, or very cheap pieces with sparse documentation.
Care & Cleaning (Avoid Abrasives, Respect Temperature Limits, UV Exposure)
Use soft sponges, avoid steel wool and knife edges, respect temperature labels, and keep items out of prolonged direct sun/UV when possible. For bakeware, an initial empty bake or two helps purge volatiles; routine care reduces both migration and wear.
How to post-cure new silicone bakeware (simple How-To)
- Wash with mild soap; air-dry.
- Bake empty at the upper end of the product’s labeled range for 45–60 minutes (avoid exceeding the label).
- Cool fully; repeat once if strong odor remains.
- Hand-wash again; now start normal use.
Reader note (real-life experience):
“After two empty bakes, the ‘new mold’ smell disappeared, and cupcakes tasted neutral again.”
Troubleshooting (quick fixes)
- Persistent odor: Repeat the post-cure; try one cycle with a tray of dry salt on the rack below to help absorb volatiles.
- Chalky or white “bloom”: Wash, then do a low-temp bake. If stickiness returns after a day, replace.
- White stress lines/tears: Retire pieces used with sharp edges or knives; repurpose as jar openers or drawer liners.
Repair, Reuse, and Responsible Disposal
Small surface scuffs aren’t a health concern, but tears or crumbs mean it’s time to repurpose (drawer grip, jar opener) or send to brand take-back if available; otherwise, dispose in trash to prevent fragments entering compost or open environments.
When Silicone Is (and Isn’t) the Best Choice
Use-Case Guidance (Bakeware, Utensils, Sealants, Medical/Infant)
Bakeware & utensils: Excellent for non-stick and high-heat use; temper first uses and avoid cutting in the pan. A single mat can replace a roll of parchment; a quality spatula can outlast several cheap plastics.
Sealants: Expect wear at seams over years; minimize scraping and clean gently to reduce fragmenting. Consider masking seams during heavy scrubbing to avoid abrasion.
Medical/infant: Prefer medical-/food-grade items from known brands; inspect and replace proactively. If whitening or tackiness appears, retire and swap.
Sustainable Alternatives to Consider (Glass, Stainless, Wood)
If you don’t need flexibility or high-heat non-stick, glass (storage/baking), stainless steel (cookware, lunchboxes), and wood/bamboo (utensils) are durable, easy to recycle or compost (for wood), and don’t shed or migrate like polymers can.
Quick alternatives table
| Material | Heat handling | Durability | Recycling/End-of-life | Notes |
| Silicone | Oven/freezer-safe; avoid open flame | Long-lived; flexible | Limited recycling; some take-backs | Great for nonstick + flexible lids/mats |
| PTFE (nonstick) liners | High heat but sensitive to scratches | Surface can wear | Not curbside recyclable | Avoid metal tools; no cutting |
| Glass | Oven, microwave (check label) | Very durable if not dropped | Widely recyclable | Best for storage/baking without flexibility |
| Stainless steel | Excellent, stovetop/oven | Extremely durable | Widely recyclable | Great for cookware/lunchboxes; not flexible |
Frequent Ask Questions

James Parker
James Parker is an environmental expert, writer, and the founder of Envirose.com. Over the years, he has dedicated his work to studying sustainable practices, renewable energy solutions, and eco-conscious lifestyles. Through Envirose, he aims to inspire individuals to make small yet powerful changes in their daily lives that can collectively create a positive impact on the planet. When he’s not writing or researching, you’ll often find him outdoors, exploring nature and finding new ways to live in harmony with it.